AAPE STEM CELL EXOSOME HAIR LOSS THERAPY
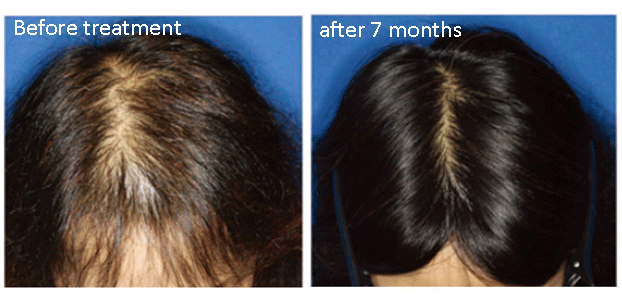
AAPE® (Advanced Adipose-Derived Stem Cell Protein Extract) hair care serum is a safe, non-invasive treatment that can be applied in a clinical setting without downtime or discomfort as a hair loss treatment.
The serum’s adipose or fat tissue content provides large numbers of mesenchymal stem cells (MSCs) which are located within the stromal vascular fraction (SVF) which contains powerful regenerative stem and stromal cells, progenitor cells, growth factors and many other powerful biologic components that work to regenerate cell growth.
AAPE® hair treatments can be used with PRP or Platelet Rich Plasma. Like PRP, recent scientific literature supports the use of adipose-derived stem cells to help enhance hair growth from weak hair follicles.
AAPE® is the first product based on stem cell described in SCI journals with clinical results in humans.
Exosomes from human adipose derived stem cells promote proliferation and migration of skin fibroblasts.
AAPE has over 600,000 clinical treatments used by over 3,000 dermatologists in more than 30 countries worldwide.
In Japan, over 200 clinics are using AAPE with a clinical hair program for hair regrowth. The brand AAPE is approved by dermatologists and medical professionals from Korea, Japan and USA.
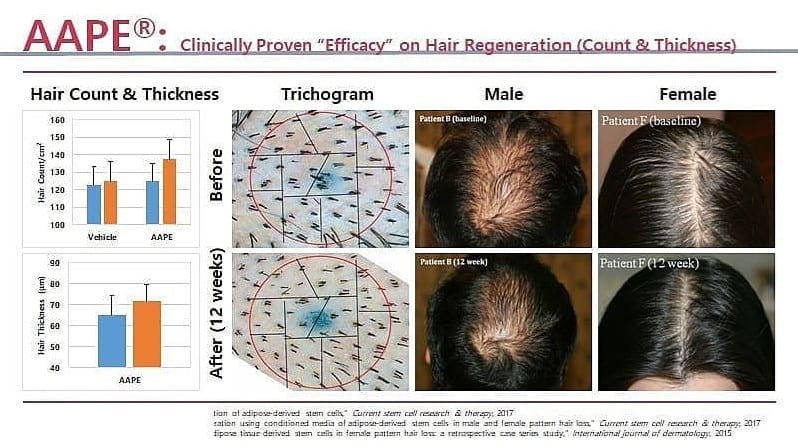
Adipose-derived stem cell-conditioned medium is rich in growth factors such as vascular endothelial growth factor, hepatocyte growth factor, platelet-derived growth factor, and insulin-like growth factor 1.
Numerous research papers in the hair growth field using Adipose-Derived Stem Cells. Papers related to ADSCs and hair on PubMed.
Treatment using adipose-derived stem cell-conditioned medium appears highly effective for alopecia and may represent a new therapy for hair regeneration. Hair Regeneration Treatment Using Adipose-Derived Stem Cell Conditioned Medium – Hirotaro Fukuoka, MD, PhD, and Hirotaka Suga, MD, PhD.
The body of evidence in favor of the use of adiposederived stem cells and ASCbased therapies for male/female pattern hair loss is steadily growing. These therapies offer the benefit of reduced side effects compared to the current treatment modalities and appear to be effective in both males and females. With relatively no new formally approved treatments for hair loss in the last 20+ years, the future of hair restoration may lie in adiposederived stem cell-based therapies. Adipose-derived stem cell-based therapies on male/female pattern hair loss
- US FDA, CFDA Approval
- PCPC US registration
- 15 International SCI journals
- 28 Patents registration worldwide
STEM CELL EXOSOME FROM AAPE
PRP VS AAPE FOR HAIR LOSS, WHICH IS BETTER?
PRP (platelet-rich plasma) therapy for hair loss is a three-step medical treatment in which a patient’s blood is drawn, processed, and then injected into the scalp. … Sometimes this approach is combined with other hair loss procedures or medications.
According to Wikipedia “Platelet-rich plasma, also known as autologous conditioned plasma, is a concentrate of platelet-rich plasma protein derived from whole blood, centrifuged to remove red blood cells. Evidence for benefit is poor.”
Because PRP therapy involves injecting your own blood into your scalp, there carries a risk of side effects such as injury to blood vessels or nerves, infection, calcification at the injection points, and scar tissue. There is also the chance that you could have a negative reaction to the anesthetic used in the therapy.
AAPE (Adipose-derived stem cells Proteins Extracts) is a mixture of refined growth factors extracted from human adipose to promote hair growth and scalp health using outstanding ingredients approved by US FDA and CFDA.
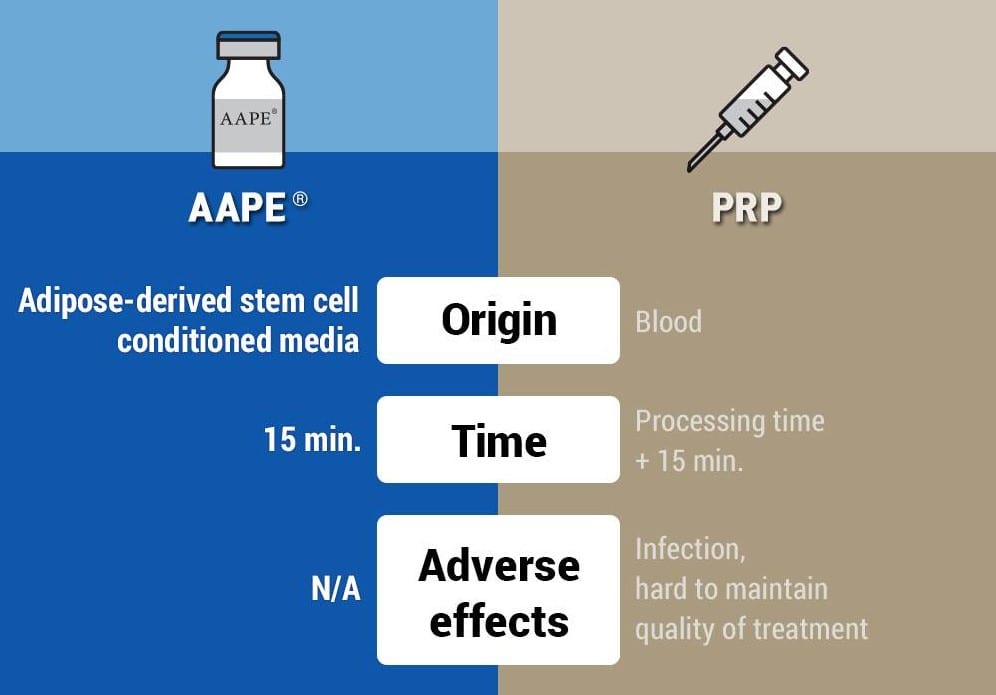
The AAPE procedure is safer, easier, cleaner, non-invasive, and painless. AAPE does not require any blood to be taken from you like PRP. There is no anesthetic required for the treatment. PRP in the best condition, contain 10-15 growth factors. AAPE in comparison, contains more than 150 growth factors.
If you are losing your hair or would like to thicken your existing hair, we invite you to give us a call at 647-492-9093 or upload your hair photos to our online assessment form here.
STEM CELL EXOSOME FROM AAPE – WHAT IS EXOSOME THERAPY?
Cellexosome® therapy using AAPE plus plant-derived exosome (panax ginseng exosomes and asparagus exosomes) is gaining popularity as one of the promising areas of regenerative treatment for hair loss. Exosome is a naturally formulated nano carrier of cell signalling. Using exosome therapy, Cellexosome® is applied topically using a micro-needling device to regrow hair due to its growth factor content. After application, Cellexosome® will trigger healing, cell stimulation and natural regeneration of hair follicles. Cellexosome® therapy is ideal for people with thinning hair, excessive shedding or hair loss. Exosomes are regenerative cells that can heal, repair, stimulate and restore cells and tissues.
AAPE Skin Ampoule
To Promote skin regeneration and wound healing by the outstanding ingredients. AAPE is a mixture of refined growth factors extracted from human adipose-derived stem cells conditioned media and induces collagen synthesis in dermal cells to maximize the revitalizing effects of your skin regeneration.
AAPE FREQUENTLY ASKED QUESTIONS
Stem cells are primal cells found in all multi-cellular organism(s) that retain the ability to renew themselves through mitotic cell division and can differentiate into a wide range of specialized cell types.
Adipose Derived Stem Cell (ADSC). Human adipocytes or “fat tissue” extracted during the process of lipoaspiration contain the maximum number of stem cells in human body.
AAPE stands for Advanced – Adipose-Derived Stem Cell – Protein – Extract
Is a mixture of refined growth factor proteins that is extracted from human adipose derived stem cells. AAPE contains about 150 growth factor proteins.
PRP (platelet-rich plasma) therapy for hair loss is a three-step medical treatment in which a patient’s blood is drawn, processed, and then injected into the scalp. … Sometimes this approach is combined with other hair loss procedures or medications.
According to Wikipedia “Platelet-rich plasma, also known as autologous conditioned plasma, is a concentrate of platelet-rich plasma protein derived from whole blood, centrifuged to remove red blood cells. Evidence for benefit is poor.”
Because PRP therapy involves injecting your own blood into your scalp, there carries a risk of side effects such as
injury to blood vessels or nerves, infection, calcification at the injection points, and scar tissue. There is also the chance that you could have a negative reaction to the anesthetic used in the therapy.
The AAPE procedure is safer, easier, cleaner, non-invasive, painless, and cheaper. AAPE does not require any blood to be taken from you like PRP. There is no anestetic required for the treatment. PRP in the best condition, contain 10-15 growth factors. AAPE in comparison, contains more than 150 growth factors.
AAPE secret potent stimulants, otherwise known as cytokines, which stimulate the growth of hair follicles. Scientific studies show that also stem cell transform into new hair follicle cells.
Our AAPE Hair procedure is administered to the scalp by use of a microneedling machine. The procedure is pain free and non-invasive. You can comfortably leave the clinic after the procedure without any downtime.
Each AAPE Hair procedure takes approximately half hour.
Each treatment is $599, however we do recommend you do 3 treatments, for 3 consecutive months. When you prepay for 3 months, you save $400, so for 3 treatments, the price is $1397.00.
You should see visible results after 3 month. Since hair grows in cycles, you will see continued hair growth improvement in the following months after the treatments.
After the initial 3 consecutive monthly treatments, you can wait for another year to do a maintenance procedure once a year afterwards.
Yes, active proteins are derived from human source and do not have allergic reactions. There have been hundreds of thousands of procedures done throughout the world using AAPE.
Adipose derived stem cell treatments can be used as a stand-alone treatment or in conjunction with other therapies for hair regrowth like PRP, laser therapy, medications, as well as hair transplantation.
A big percentage of hair loss, about 95%, is either male pattern or female pattern hair loss. 30% of women under the age of 50 have female pattern hair loss while 50% of women over the age of 50 experience it. The most common diagnosis in hair loss is androgenetic alopecia which is manifested with patients having thick hair progressing to thinner hair. AAPE is ideal for both men and women.
HAIR REGENERATION THERAPY: APPLICATION OF ADIPOSE-DERIVED STEM CELLS
Various cytokines secreted by adipose-derived stem cells (ADSCs) have been reported to play a key role in angiogenesis and wound healing, as well as to stimulate hair follicles and induce hair growth. We confirmed that the effects of adipose-derived stem cell-conditioned medium (ADSC-CM) containing multiple growth factors on hair growth using both in vitro and in vivo experiments, developed a hair regeneration therapy that involves local administration of a commercialized ADSC-CM formulation (advanced adipose-derived stem cell protein extract, AAPE®😉 and applied this therapeutic technique in clinical settings.
AAPE® is a formulation that contains lyophilized forms of proteins secreted from cultured ADSC collected and isolated from healthy adult women. This product was examined for possible bacterial and viral infections, as described previously. During production, ADSC are maintained in a low-oxygen environment (2% O2, 5% CO2, 93% N2) for 72 hours to induce cytokine secretion. The product contains numerous cytokines, including vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), keratinocyte growth factor (KGF) and platelet-derived growth factor (PDGF). When these cytokines are in powdered form, their activities are not diminished, and consequently, they can be stored for long periods. Cytokines are dissolved in 4 ml of saline per vial immediately prior to usage.
Regardless of gender, individuals with alopecia of all levels of severity are eligible to receive treatment. According to our experience, in individuals with alopecia affecting nearly the entire head, those who have been bald for a long time, and the elderly, the duration of treatment is often prolonged and the effects may be limited due to atrophy of hair follicles and progression of fibrosis. Such details are explained to patients in advance, and treatments are then administered should they wish to receive them. If inflammatory skin disorders are observed, treatment is given after inflammation has subsided.
The efficacy of hair regeneration therapy, as well as its side effects and safety, is thoroughly explained to each patient, and treatment is performed upon obtaining written consent. For a single treatment, 3-4 ml of AAPE® is administered to the entire scalp using the papule injection (intradermal injection). A 31-gauge needle is used to inject a volume of roughly 0.02 ml into each injection site, which are spaced 1 cm apart. In most cases, AAPE® is administered in combination with vitamin B1 (5 mg), vitamin B6 (2.5-5 mg), vitamin H (1 mg), vitamin C (80-100 mg), vitamin E (5 mg), coenzyme Q10 (10 U) and amino acids as nap page mesotherapy, in order to improve the function of mesotherapy with antioxidant effects and induce hair growth. We refer to this combined therapy as hair regenerative therapy (HARG®) enhanced by hypoxic ADSC-CM. Although it is possible to receive treatment without anesthesia, patients who complain of pain are given topical anesthesia with lidocaine or anesthesia that blocks the supraorbital nerve and greater occipital nerve. Typically, injections are given once a month and are repeated 6-8 times. Treatments are performed ≥10 times in some patients, depending on the severity of alopecia or the patient’s treatment goals. In addition, some men also take finasteride as combination therapy during treatment or as maintenance therapy after treatment completion.

A 31-gauge needle, whose length is controllable with a screw guide (Feel-Tech Co., Ltd., Busan, South Korea), is used for intradermal injection with an adapter locking to syringe (Medikan Co., Ltd., Seoul, South Korea).
Cytokines injected into the scalp are considered to act on local cells immediately after administration. However, our clinical experience suggests that it takes time for these cytokines to promote proliferation and protein synthesis on the scalp, and for such effects to become visible. We describe the general course of our treatment for androgenetic alopecia (AGA) and female pattern hair loss (FPHL), although the degree and speed of improvement vary between individuals.
After the initial 2-3 treatments (2-3 months after first treatment), new growth of thin hair is observed on the scalp by trichogram, while patients rarely notice any changes at this point. We marked the shaved area with tattoos on the top of the head, and counted number of hairs in the same area over time using a trichogram in 21 patients (16 AGA and 5 FPHL; age range: 27-69 years) who consented to these procedures. The number of hairs at 3 months after the first treatment increased significantly in comparison with the number of hairs before treatment (141.3±31.4, 109.8±43.5, respectively; P < 0.01).
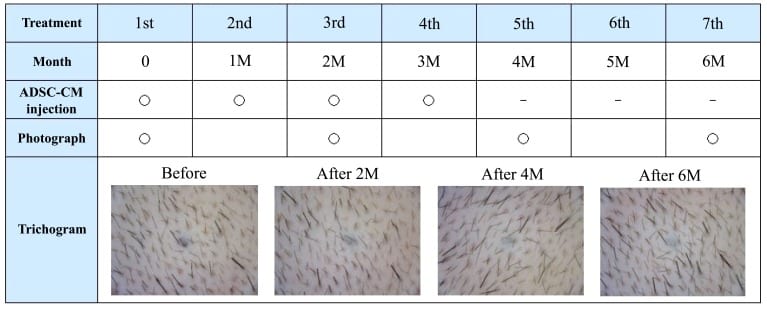
A table showing the time flow of our treatment. Patients undergo multiple courses of ADSC-CM (AAPE) injections once a month. Two or three months after the first treatment, new growth of thin hair is observed on the scalp by trichogram. This case is 4 treatments (injections) of AAPE.
With an increasing number of treatments, new hair growth and the number of hairs in the anagen phase increases, and such effects become noticeable to patients. We reported that patient satisfaction scores measured by the visual analog scale increased with the number of treatments, which targeted AGA and FPHL. After 4-5 treatments (4-5 months after first treatment), patients are often able to notice changes in hair quality or to confirm reductions of area in the extent of thinning hair through photograph. In our experience with AGA treatment, hairline improvement follows parietal improvement. We have treated some patients who wanted additional improvement after hair transplantation for frontal baldness at another clinic. Even though they were likely to need repeated treatments, we confirmed the efficacy of our treatment.
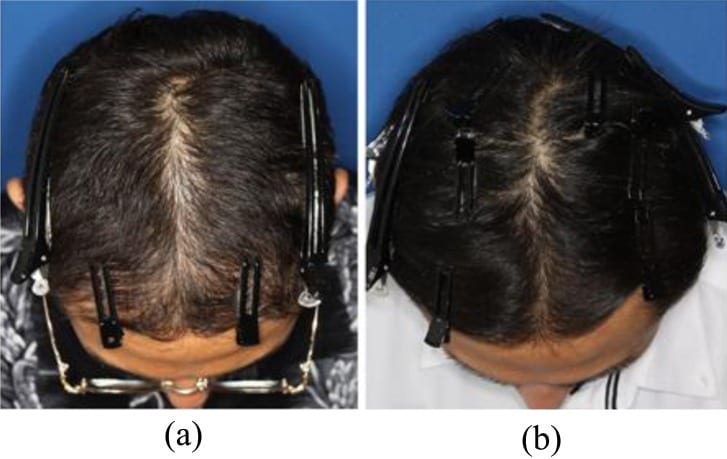
Photographs of 35-year old male patient, on two occasions, 500 follicular units had been transplanted at another clinic in the frontal area (at age 25 and 29 years), on 1 mg/day finasteride, starting 5 years before initial treatment. (a) Before treatment. (b) After 8 sessions of AAPE treatment (1 month after final treatment). Marked hair growth was observed after treatment.
In order to obtain additional clear evidence regarding the effects of our hair regeneration therapy, after providing a thorough explanation and obtaining consent, we conducted a half-side comparison test in 10 patients (8 AGA and 2 FPHL; age range: 20-73 years). Without oral finasteride, normal treatment was given 6 times on one side, and the same volume of placebo (saline) was injected on the opposite side. First, the intersection of a line that connects both ears at the top of the head and a line extends from each lateral canthus in the cranial direction are marked with tattoos. Subsequently, the number of hairs within both circles (11 mm diameter, 95 mm2 area) with the tattooed markings at the center was quantified by trichogram before and after the treatment. Although the total number of hairs increased on both the treatment and placebo sides after 6 months of treatment, the increase over the treatment period was significantly different between the treatment and placebo sides (increase in hair count; 18.4±9.4, 6.5±11.7, respectively; P < 0.01). After completion of this test, normal treatment started on the placebo side and none of the patients showed any obvious bilateral differences in the end.
Although we have to date performed treatment in over 1,000 patients, we have not experienced complications such as AAPE®-induced allergic reactions or infection. None of the patients experienced sustained injection pain, chronic headache or itching sensations in the scalp. Although it has been reported that tissue necrosis and cicatricial baldness can occur after the mesotherapy procedure, such serious complications have not occurred after our treatments.
Although treatment with the present method accompanies pain at the time of injection, it maintains a high level of safety and is less invasive when compared to treatments that use hair transplantation or autologous stem cells. For these reasons, the present treatment method is becoming more common among individuals who do not show sufficient effects with medications such as oral finasteride and topical minoxidil or among those with female pattern hair loss, and is already in use at many facilities. However, with regard to treatment outcomes, there are no large-scale studies similar to the finasteride study and evidence remains poor. In the future, a high-quality comparison study is needed.
In our experience, most patients exhibited improvements. However, there are individual differences in the degree of improvement. Even if improvement is objectively evident, sufficient satisfaction is not obtained in all patients because treatment expectations increase with improvement. In addition, although ADSC-CM is considered safe because it maintains a physiological ratio, there may be a particular composition of cytokines specific to hair regrowth that also ensures safety. We believe that studies to optimize cytokine composition with ADSC-CM will make our treatments more effective.